Mimicking the micro-environment in organoids
In order to better understand the pathophysiology of cancer biology, there is a growing need for experimental in vitro models that can recapitulate not only the characteristics of tumors, but also the surrounding microenvironment of the tumor. The role of the extracellular matrix (ECM) is particularly important in the pancreatic ductal adenocarcinoma where the ECM component, stroma contributes to 80% of the tumour volume. Thus, there is a need to develop model systems that can effectively recapitulate the stromal environment to investigate the development of therapeutics.
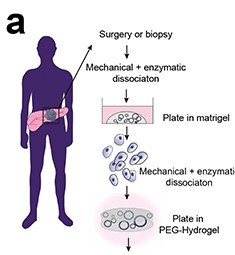
Figure 1: Schematic of establishing pancreatic ductal organoids in PEG-hydrogel (Image from Below et al, 2022)
While organoid models have gained traction recently to study human cancers, many of the organoid models are embedded in animal-derived hydrogels that are associated with batch-to-batch variability and an ill-defined composition. This leads to failure in replicating the ECM in human cancers. In addition, there is a need to develop matrices that can equally support both epithelial organoids and stromal cells. In a recent study published in Nature Materials, researchers developed a synthetic 3D hydrogel extracellular matrix which can simulate the traits of a pancreatic microenvironment, tumour-ECM interactions, and tissue stiffness that could support the growth of organoids developed from human tissue samples.
To replicate the ECM-receptor interactions, the researchers performed a proteomic analysis of normal and cancerous pancreatic tissue to build a reference set of matrisomal proteins. They further tested these observations using functional analysis where cell culture dishes were pre-coated with ECM ligands identified in the proteomic analysis and adhesion kinetics was performed for tumour and stromal cells.
The interactions between the specific ECM components, laminin 511/521 and integrins α6/α3 subunits, were replicated in this scaffold to reveal the integral role of laminin and integrin in the establishment and survival of these organoids. This hydrogel also captured the stiffness ranges that were observed in the murine and human pancreatic cancers. The tissue stiffness was simulated by adjusting the properties of the hydrogel and altering organoid growth. Stromal cells of the pancreas were also incorporated into this hydrogel scaffold to replicate the traits of a tumor microenvironment in vivo, thus presenting a model which extensively mimics pancreatic cancer tumor microenvironment. This will allow studies using normal and pancreatic cancer cells to be carried out in a system which provides growth control and can support basic and translational studies of human pancreatic cancer.
