Microscopy tools for imaging microphysiological systems
Surat Parvatam
A key element to enable an increase in the usage of organ-on-chip (OOC) devices in biomedical research and drug discovery is the ability to monitor and measure quantitative information from these systems at cellular level. Non-invasive imaging methods also help in long-term and live imaging of cells in OOC devices. In the recent decade, several new imaging methods have been designed which are beginning to be used to assess various parameters in OOC devices. In this article, we explore the different categories of imaging techniques to exploit OOC models. Figure 1 provides an overview of various imaging modules currently available.
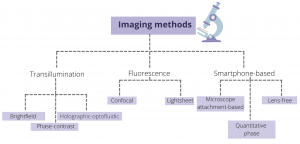
Figure 1: An overview of various imaging tools for probing OOCs
Transillumination is one of the standard imaging modules and involves light being transmitted through the sample on a platform. In this setup, a light source is placed on one end of the platform, while a detector is placed on the other end. Three common forms of transillumination method include brightfield, phase-contrast, and holographic optofluidic microscopy. Brightfield microscopy is one of simplest optical illumination techniques where the sample is illuminated from below using white light and observed from above, and contrast is generated due to attenuated light in the denser areas of the sample. When light travels through a medium, its amplitude and phase changes due to the properties of the medium. These phase changes can convey the important structural information about the sample, which may not be captured in brightfield imaging, but can be made visible using phase contrast microscopy. Optofluidics is an emerging field that combines tools of optics with microfluidics. In this set-up, the sample flowing within a microfluidic channel is illuminated using a partially coherent light. The light scattered by the sample interferes with the background light to create a hologram on a digital sensor which is placed directly beneath the microfluidic channel.
Unlike transillumination methods, fluorescence imaging requires the conjugation of fluorophore to the sample to obtain an image. In a wide-field fluorescence microscopy, the entire sample is flooded with the light source leading to excitation of the fluorophore in all parts of the sample. This often leads to detection of a large amount of background light in the sensor. To avoid this, confocal microscopes use a point illumination method where only a single point is illuminated at one time. In addition, it uses a pinhole in front of the detector that helps to capture the light from a single plane rather than the entire sample depth. Both of these features help to image samples at high resolution and greater depth. Another high-resolution fluorescence microscopic technique is light sheet microscopy where only a thin slice of the sample (few hundred nm to few mm) is illuminated using a laser light-sheet (a laser focussed in one direction using a cylindrical lens). Both confocal and light sheet microscopy illuminate only a very small part of the sample at a time, leading to increased signal-to-noise ratio, reduced photodamage, and increased resolution.
Another form of imaging that has risen in the past few years is the smart phone-based imaging where the basic set-up includes light source, smartphone, and the microfluidic device. The light travels through the sample in the microfluidic device and hits the detector that is present in the smartphone to create the image. While this method is low-cost and provides ease of access, it is limited by the imaging application on the smartphone. In addition, various image adjustments, such as spatial light bias adjustment or localized focussing and defocussing may be inappropriate to image scientific images.
The decision to choose a specific microscopic tool depends on various factors, some methods are focussed on greater resolution, while others have lower cost and better ease of access. Thus, informed decisions can help in the optimum exploitation of the OOC.
