Organs-on-chips are 3D microfluidic devices that are lined with living human cells and aim to capture mechanics and physiological response of particular organ or a system of organs (watch our video in this newsletter on how lungs-on-a-chip are being for developing COVID-19 therapeutics). While these organ chips have gained tremendous traction in the past decade, there are certain challenges that still prevent the widespread adoption of these models in the industry for drug discovery, disease modelling and personalized medicine.
A recent survey attempted to understand the broad challenges for translation and commercialization of this technology by the various stakeholders
Fig 1: Stakeholders included in the survey
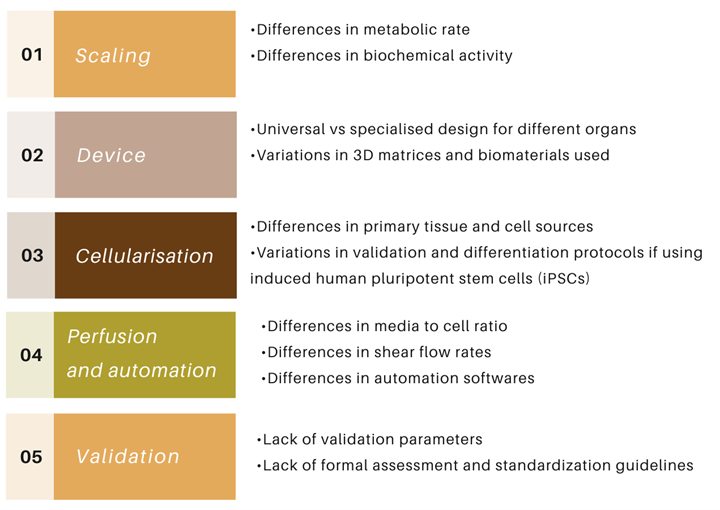
Fig 2: Sources of variabilities hindering wide-spread adoption of organ-on-chip models in industry
The survey highlighted several parameters that need to be considered during the development and design of an organ-on-chip: design of the chip itself, material of the chip and 3D matrices, source of cells used in these devices, differentiation protocols when using induced pluripotent stem cells, type and density of media, the flow rates, and validation protocols.
For example, different developers can employ different designs of organ chip device, use different substrates and biomaterials. Polydimethylsiloxane (PDMS) is one of the most widely used 3D matrix, due to its non-toxic and flexible nature. However, its propensity to absorb tracers, drugs, and toxicants is a significant drawback which is leading to developers exploring new matrices and substrates. The source of cells can also contribute to a huge amount of variability. Particularly, iPSCs often exhibit an immature phenotype and require validation of differentiation methods due to line-to-line variability, and complex differentiation protocols can further add to heterogeneity.
Different developers can use different rates of perfusion of media which can lead to differences in nutrient consumption, waste production, shear flow stress, and diffusion kinetics across membranes. Validating these models in another area which is a point of hesitancy amongst the industry stakeholders. As these models are based on human cells and human biology, validating the results obtained from these models using animal models is a highly debatable idea. Whereas, the idea of validating these systems against human clinical samples and human-relevant data is gaining ground.
However, there are no guidelines or standardization protocols that are in place to address these points which bring about site-to-site and batch-to-batch variabilities.
Many of the end-users felt that having detailed guidelines and standards for each of these parameters is necessary to ensure robustness and reproducibility in organ-on-chip. This in turn would increase trust in these systems, leading to wider adoption by industry and other end-users.
Reference: Allwardt, V. et al. Translational Roadmap for the Organs-on-a-Chip Industry toward Broad Adoption. Bioengineering 2020, 7, 112. https://doi.org/10.3390/bioengineering7030112