Microphysiological systems have been rapidly advancing in the last few years, bringing about a paradigm change in the areas of diagnostics, disease prevention and treatment. The use of these systems is also rapidly advancing in other sectors, such as cosmetics, food, medtech, etc. However, many of these industries often work in siloes, despite the fact the common innovations could drive the entire field ahead.
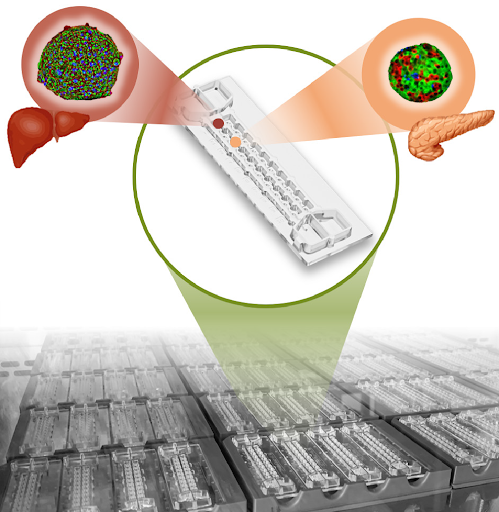
A recent meeting brought an interesting set of multi-disciplinary fields together that were working on MPS models to promote cross-industry collaborations. One group was working on engineering scalable multi-tissue models where several organ models are interlinked to study the effects of various drug candidate molecules. To create a scalable system, the microfluidic platform was produced by a mass fabrication process, and spheroids were loaded into the system using an automated robotic pick and place transfer that enables mass multi-tissue experiments.
Another group was working on generating precision tools and digitisation softwares to generate automation which would be critical factor for the use of these systems in industrial and clinical applications. Deep learning algorithms could be a game-changer for high-throughput organoid sorting. Similarly, high resolution imaging modules could be coupled with deep learning to classify different stages and types of the organoids during the development process.
3D printing is another area which has a lot of scope for cross-sectoral collaboration. While traditional 3D printers work in a layer-by-layer manner, recently tomographic printers have been introduced that create entire object volume in very small span of time (~30s). 3D printing biostructures can further contribute to scalability and reducing contamination as the entire process can be done in a sealed, sterile environment.
Figure 1: Microfluidic system with interconnected liver and islet tissues(Kopanska and Rimann, 2002, re-used under CC license)
The meeting also hosted two groups that were working on cellular agriculture and cultivated meat, that are other rapidly advancing areas. The current procedure to produce muscle fibres relies on differentiation of myoblasts (myogenic progenitors). However, myoblasts often display limited proliferation and differentiation capacities. Studies have shown that overexpressing certain transcription factors can convert mouse fibroblasts in to induced myogenic progenitor cells (iMPCs). These myogenic cultures include a mix of muscle fibres and progenitor cells that can expand for longer term without losing their self-renewal capabilities. This system is now being understood to devise a similar strategy to improve cow myoblast differentiation for cultivated meat.
These examples show the advances being made in various sectors of industry that involve organotypic models. While each of these advances is creating waves in their respective areas, these advances also have broader potential implications for how innovations in basic research, translational applications, medical devices, food etc could interface and facilitate each other. This could in turn lead to wide-spread exploitation and advancement, rather than incremental rate of growth of these technologies.